The National Agency for Food and Drugs Administration and Control (NAFDAC) has issued a public notification concerning discovering a falsified Coco Samba Herbal Mixture 100ml in circulation.
This product was scrutinized after being seized by German customs and subsequently analyzed by the German Official Medicines Control Laboratory (OMCL).
The report from the analysis revealed that the product contained undeclared amounts of sildenafil at 150mg, exceeding the maximum daily dose for sildenafil. Sildenafil is a medicine prescribed for the treatment of erectile dysfunction and pulmonary arterial hypertension (PAH).
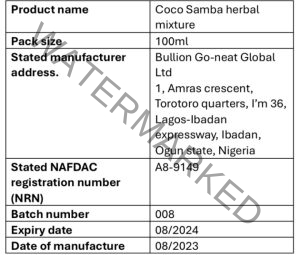
Further investigations uncovered that the NAFDAC Registration Number on the product “A4-9149” is falsified, differing from the genuine product’s registration number obtained from the NAFDAC database, which is “A8-4418L.” NAFDAC’s laboratory analysis corroborated the presence of undeclared sildenafil content.
Falsified product details
What you should know:
Sildenafil may interact with nitrates found in some prescription drugs, potentially causing a significant drop in blood pressure that could be life-threatening.
Individuals with diabetes, high blood pressure, high cholesterol, or heart disease, who often take nitrates, are particularly at risk.
While the falsified product was intercepted in Germany, there is a possibility that it may have been distributed within the country through informal channels.
It is crucial to detect and remove it from circulation promptly to prevent harm to consumers.
NAFDAC urges importers, distributors, retailers, and healthcare providers to exercise caution and vigilance within the supply chain, avoiding the importation, distribution, sale, and administration or use of falsified or substandard medicinal products.
All medical products must be obtained from authorized and licensed suppliers, with a thorough check of authenticity and physical condition.
Members of the public in possession of the mentioned product are advised to discontinue its sale or use and submit the stock to the nearest NAFDAC office.
It is essential not to use this falsified product. Individuals who have used the product or experienced any adverse reactions or events after use are advised to seek immediate medical advice from a qualified healthcare professional.